Why does ice melt?
- Подробности
- 2
Ice melts when it absorbs heat and changes from solid to liquid.
Why does ice melt?
How ice melts
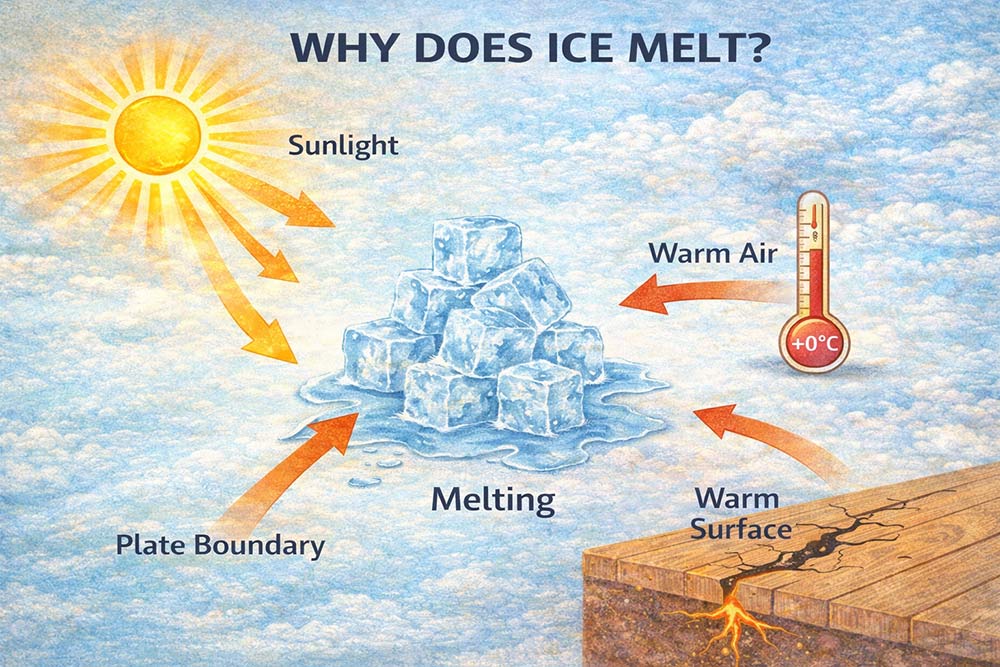
Ice melts because it absorbs heat from the surrounding environment. Ice is frozen water, and when the temperature rises above 0 °C, it begins to change its state.
When ice gets warmer, its molecules start to move faster. The solid structure of ice breaks apart, and it turns into liquid water. This process is called melting.
Heat can come from the Sun, warm air, or contact with a warm surface. The more heat the ice absorbs, the faster it melts.
That is why ice melts on a warm day or inside a warm room.
LISTEN TO THE TEXT